Stimulation of the natural hair cycle with fractional non-ablative Er:YAG laser
Dr Iva Talaber
Androgenetic alopecia is the most prevalent hair-loss disorder in men. It can exert a profoundly negative eff ect on identity and self-image. With different pharmacological, surgical, light-based, and nutraceutical treatments available, selecting the most suitable therapy can be challenging.
Androgenetic alopecia (AGA) or male pattern baldness, is a multifactorial disorder caused by genetic factors, hor-monal dysregulation, environmental and systemic factors, and aging (1). AGA is estimated to aff ect 50% of Caucasian males and females by the ages of 50 and 80, respectively (2). The prevalence and severity of AGA in Asian and African ethnicities is lower than in Caucasians (3,4). The incidence of AGA is increasing with age in both genders across all ethnicities(5). As hair is an important component of identity and self-image, patients with androgenetic alopecia may experience a distorted body image and a decrease in quality of life (6,7), with early-age onset being especially important as a possible source of depression in young adults (8). Available treatment options for AGA include diff erent oral and topical pharmaceuticals, nutraceuticals, laser therapy, platelet-rich plasma (PRP) and a variety of surgical transplant procedures (9).
The pharmaceuticals include two FDA-approved drugs, oral finasteride and topical minoxidil. Many patients seek alternatives to pharmacological treatment, which can present a plateauing of the response as well as undesirable side effects (8). AGA is characterised by a gradual and progressive miniaturisation of hair follicles, accompanied by a decrease in the ratio of the active growth to the resting stages of the hair cycle (8). Hair follicles are rich in stem cells, which regenerate in continuous cycles consisting of three stages: growth (anagen), involution (catagen), and rest (telogen), all of which are largely aff ected by the Wnt/β-catenin signal-ling pathway (10,11).
The dermal papilla of the hair follicle is a major regulator of the hair cycle, including progenitor cell activation (10). Interestingly, the upregulation of Wnt/β-catenin signalling pathways has been indicated in several of the AGA treatment options, with a demonstrated efficacy, including minoxidil(12), PRP (13,14) and laser treatment (15). Numerous studies have demonstrated efficacy of laser therapy for AGA (2,10,16) and an absence of adverse effects (17,18). Whereas low-level laser therapy utilises photobiomodulation mechanisms to induce cellular metabolism,(19) high-energy medical lasers induce tissue regeneration through photothermal effects (9,10).
Laser hair regrowth treatment is associated with minimal risk and potentially better outco-mes compared to standard pharmacological treatment (2,9). Studies on murine models suggest that laser irradiation aff ects the hair cycle by promoting telogen-to-anagen tran-sitions (20) in both non-ablative (1550 nm erbium-glass (20)) and ablative treatment (2940 nm erbium-YAG15 and 10600 nm CO2 laser (21)). In addition, the laser energy acts by increasing blood flow at the dermal papilla (22). Both ablative and non-ablative lasers have already been successfully used in monotherapy to treat androgenetic alopecia in men and women (1540 nm Er:glass23, 1550 nm Er:glass (20,24,25), 2940 nm Er:YAG26–28, 1927 nm thulium (29) and 10600 nm CO221 laser).
In recent years combining laser therapy with other modalities, such as pharmaceu-ticals (30–33), exosomes and PRP (29) is on the rise. Fotona’s HAIRestart® laser therapy relies on the 2940 nm Er:YAG wavelength to perform a non-ablative procedure using a patented SMOOTH™ mode, consisting of trains of sub-ablative laser pulses. The advantage of Er:YAG laser used in SMOOTH™ mode is that laser light is absorbed in the most superfi cial (<10 μm) layer of the skin, with only heat diff using to the deeper layers, making it a very safe form of energy, which is especially important when treating the scalp.
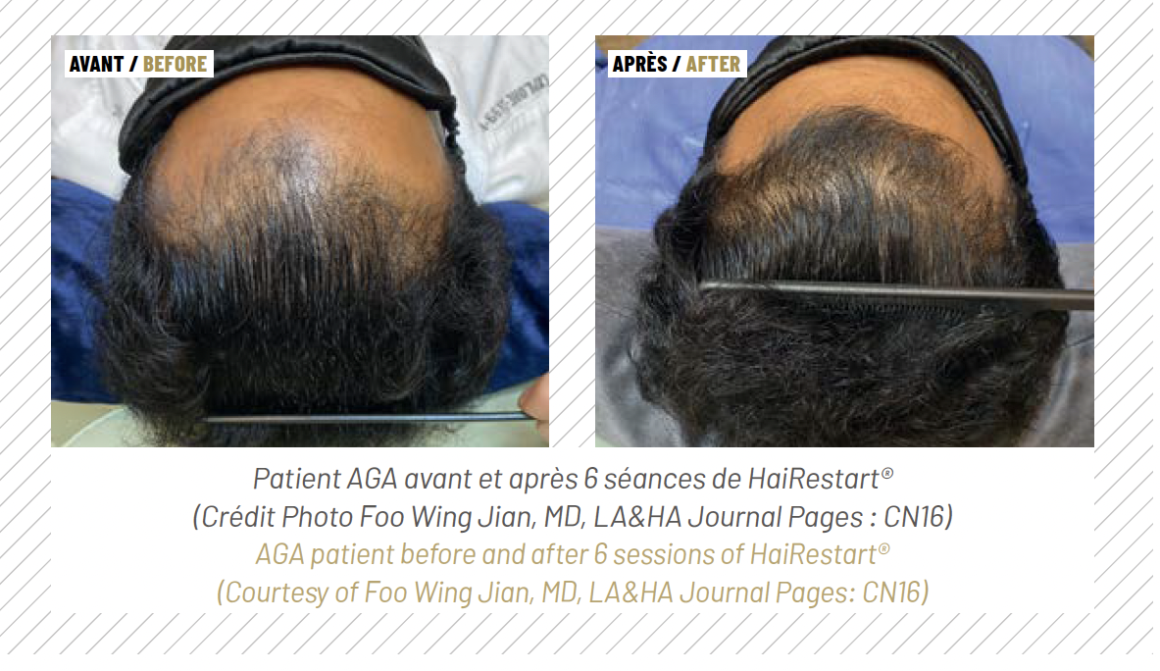
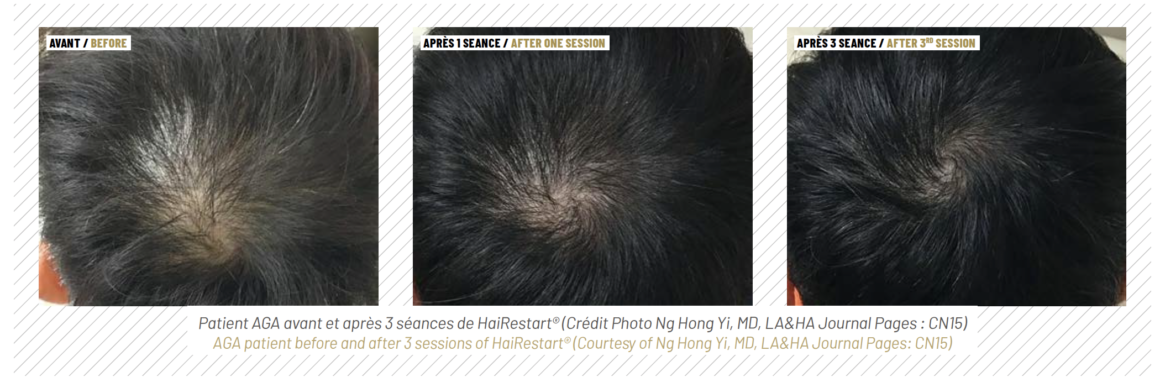
The heat pulses penetrate the skin to approximately 0.5 mm in depth, resulting in tissue hyperthermia as well as in paracrine signaling that activates fibroblasts to initiate regenerative processes in the skin (34,35). The HAIRestart® therapy’s effects on the scalp include improved blood supply to the hair follicles (22), stimulation of the hair cycle by promoting faster telogen-to-anagen transitions (20), an increase in hair density and thickness (36) and prevention of further hair loss (26). HAIRestart® is suitable as an alternative to pharmaceuti-cals in the form of a monotherapy, as an adjunct treatment to other modalities, to stabilise hair thinning before a hair transplant, or as maintenance treatment. This unique versatility allows it to fill several niches in AGA therapy.
Dr. Iva Talaber

PhD, is a Clinical Affairs Associate at Fotona. She collaborates with doctors and healthcare professionals on clinical studies of the Er:YAG laser for hair stimulation and assists in developing the HAIRestart® protocol.
References
1. Cuevas-Diaz Duran, R. et al. The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. Int. J. Mol. Sci. 25, (2024).
2. Afifi , L. et al. Low-level laser therapy as a treatment for androgenetic alopecia. Lasers Surg. Med. 49, 27–39 (2017).
3. Tanaka, Y., Aso, T., Ono, J., Hosoi, R. & Kaneko, T. Androgenetic Alopecia Treatment in Asian Men. J. Clin. Aesthet. Dermatol. 11, 32–35 (2018).
4. Wang, T. L. et al. Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br. J. Dermatol. 162, 843–847 (2010).5. Fan, S. M. Y., Cheng, Y. P., Lee, M. Y., Lin, S. J. & Chiu, H. Y. Eff icacy and safety of a low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, self-comparison, sham device-controlled trial. Dermatologic Surg. 44, 1411–1420 (2018).
6. Han, S.-H. et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann. Dermatol. 24, 311–318 (2012).7. Cash, T. F. The psychological eff ects of androgenetic alopecia in men. J. Am. Acad. Dermatol. 26, 926–931 (1992).
8. Sonthalia, S. Hair Restoration in Androgenetic Alopecia: Looking Beyond Minoxidil, Finasteride and Hair Transplantation. J. Cosmetol. Trichology 02, (2016).
9. Dabek, R. J., Austen, W. G. & Bojovic, B. Laser-assisted Hair Regrowth: Fractional Laser Modalities for the Treatment of Androgenic Alopecia. Plast. Reconstr. surgery. Glob. open 7, e2157 (2019).
10. Perper, M., Aldahan, A. S., Fayne, R. A., Emerson, C. P. & Nouri, K. Efficacy of fractional lasers in treating alopecia: a literature review. Lasers Med. Sci. 32, 1919–1925 (2017).11. Kitagawa, T. et al. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J. Clin. Endocrinol. Metab. 94, 1288–1294 (2009).
12. Kwack, M. H., Kang, B. M., Kim, M. K., Kim, J. C. & Sung, Y. K. Minoxidil activates -catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation eff ect. J. Dermatol. Sci. 62, 154–159 (2011).
13. Gentile, P. & Garcovich, S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil®, fi nasteride®, and adult stem cell-based therapy. Int. J. Mol. Sci. 21, 1–26 (2020).
14. Zheng, Z. et al. Up-regulation of fi broblast growth factor (FGF) 9 expression and FGF-WNT/-catenin signaling in laser-induced wound healing. Wound Repair Regen. 22, 660–665 (2014).
15. Ke, J. et al. Erbium: YAG laser (2,940 nm) treatment stimulates hair growth through upregulating Wnt 10b and -catenin expression in C57BL/6 mice. Int. J. Clin. Exp. Med. 8, 20883–20889 (2015).
16. Zarei, M., Wikramanayake, T. C., Falto-Aizpurua, L., Schachner, L. A. & Jimenez, J. J. Low level laser therapy and hair regrowth: an evidence-based review. Lasers in Medical Science vol. 31 363–371 (2016).17. Katzer, T., Leite Junior, A., Beck, R. & da Silva, C. Physiopathology and current treatments of androgenetic alopecia: Going beyond androgens and anti-androgens. Dermatol. Ther. 32, e13059 (2019).
18. Liu, K.-H., Liu, D., Chen, Y.-T. & Chin, S.-Y. Comparative eff ectiveness of low-level laser therapy for adult androgenic alopecia: a system review and meta-analysis of randomized controlled trials. Lasers Med. Sci. (2019) doi:10.1007/s10103-019-02723-6.
19. Avci, P., Gupta, G. K., Clark, J., Wikonkal, N. & Hamblin, M. R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 46, 144–151 (2014).
20. Kim, W.-S. et al. Fractional photothermolysis laser treatment of male pattern hair loss. Dermatologic Surg. Off . Publ. Am. Soc. Dermatologic Surg. [et al.] 37, 41–51 (2011).
21. Bae, J. M., Jung, H. M., Goo, B. & Park, Y. M. Hair regrowth through wound healing process after ablative fractional laser treatment in a murine model. Lasers Surg. Med. 47, 433–440 (2015).
22. Avram, M. R., Leonard, R. T., Epstein, E. S., Williams, J. L. & Bauman, A. J. The current role of laser/light sources in the treatment of male and female pattern hair loss. Journal of Cosmetic and Laser Therapy vol. 9 27–28 (2007).
23. Alhattab, M. K., AL Abdullah, M. J., Al-janabi, M. H., Aljanaby, W. A. & Alwakeel, H. A. The eff ect of 1540-nm fractional erbium-glass laser in the treatment of androgenic alopecia. J. Cosmet. Dermatol. 19, 878–883 (2020).
24. Lee, G. Y., Lee, S. J. & Kim, W. S. The eff ect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J. Eur. Acad. Dermatology Venereol. 25, 1450–1454 (2011).
25. Meephansan, J., Ungpraphakorn, N., Ponnikorn, S., Suchonwanit, P. & Poovorawan, Y. Eff icacy of 1,550-nm erbium-glass fractional laser treatment and its eff ect on the expression of insulin-like growth factor 1 and Wnt/b-catenin in androgenetic alopecia. Dermatologic Surg. 44, 1295–1303 (2018).
26. Day, D., Mccarthy, M. & Talaber, I. Non-ablative Er:YAG laser is an eff ective tool in the treatment arsenal of androgenetic alopecia. J. Cosmet. Dermatol. 00, 1–8 (2021).
27. Dai, R., Yang, X., Su, Y. & Wu, X. Eff ectiveness and safety of the ablative fractional 2940-nm Er: YAG laser for the treatment of androgenetic alopecia. Lasers Med. Sci. 39, 128 (2024).
28. Su, Y.-P. & Wu, X.-J. Ablative 2940 nm Er: YAG fractional laser for male androgenetic alopecia. Dermatologic therapy vol. 35 e15801 (2022).
29. Cho, S. Bin et al. Therapeutic eff icacy and safety of a 1927-nm fractionated thulium laser on pattern hair loss: an evaluator-blinded, split-scalp study. Lasers Med. Sci. 33, 851–859 (2018).
30. Esmat, S. M. et al. Low level light-minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: A randomized controlled study. Lasers Surg. Med. 49, 835–843 (2017).
31. Suchonwanit, P., Rojhirunsakool, S. & Khunkhet, S. A randomized, investigator-blinded, controlled, split-scalp study of the eff icacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med. Sci. 34, 1857–1864 (2019).
32. Huang, Y., Zhuo, F. & Li, L. Enhancing hair growth in male androgenetic alopecia by a combination of fractional CO2 laser therapy and hair growth factors. Lasers Med. Sci. 32, 1711–1718 (2017).
33. Cohen, P. R. Laser-assisted drug delivery for the treatment of androgenetic alopecia: ablative laser fractional photothermolysis to enhance cutaneous topical delivery of platelet-rich plasma – with or without concurrent bimatoprost and/or minoxidil. Dermatol. Online J. 25, (2019).
34. Lukač, M., Lozar, A., Perhavec, T. & Bajd, F. Variable heat shock response model for medical laser procedures. Lasers Med. Sci. 34, 1147–1158 (2019).
35. Shanina, N. A., Patrushev, A. V & Zorman, A. Histological and Immunohistochemical Changes in Facial Skin Treated with Combined Ablative and NonAblative Laser Therapy. J. Cosmet. Dermatol. (2021).
36. Dekeyser, B. Non-ablative Er:YAG-laser treatment of female patterned hair loss. PMFA J. 8, (2021).